
Liraglutide (brand name: Saxenda®) is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) that is indicated, alongside lifestyle measures, for weight management in adults who have obesity or overweight with at least one weight-related comorbidity.
This MedCase describes how to start liraglutide and support patients with weight loss in a general practice setting.
Mrs B is a 45-year-old woman who attends to discuss her weight. She is a busy working mother of three children aged 2, 4 and 6 years. She has been overweight since her teens and has a long history of trying different diets, which have brought short-term weight loss. However, she finds it difficult to stick to one single diet regime long-term and has always ended up regaining the lost weight.
She now feels she is heavier than ever and wants to take a sustainable approach to weight management.
Medically, Mrs B has a history of constipation for which she takes psyllium husks and lactulose as needed.
She saw you recently for fatigue and low mood. She was not depressed but you asked her to incorporate some gentle exercise as a means of improving her mood, though she struggled to find time to do this regularly. You ordered blood tests, which showed elevated GGT of 117 and elevated total cholesterol and low-density lipoprotein (LDL) cholesterol with a normal ratio, and HbA1c 47 mmol/mol. Mrs B takes no other regular medications.
Today, her weight is 87kg, height 160cm and body mass index (BMI) is 34. Her waist circumference is 92cm. Her blood pressure is 142/78 mmHg and you note that recent blood pressure measurements have consistently shown a systolic blood pressure in the 130-140 mmHg range. She has acanthosis nigricans and several skin tags. There are no other abnormal findings on examination.
Mrs’s B’s experience of unsuccessful yo-yo dieting is typical of many patients with obesity, who are at risk of developing one of the many associated metabolic complications. She already shows signs of these, including:
- hypertension
- insulin resistance and prediabetes
- dyslipidaemia (high LDLc)
- deranged LFTs consistent with fatty liver
- fatigue, possibly secondary to obstructive sleep apnoea
- low mood.
What can you offer Mrs B to support her weight loss?
Obesity-related complications generally increase in prevalence as BMI increases; conversely, losing 5-10% of body weight can significantly reduce complications and comorbidities.
Lifestyle interventions focused on eating habits and physical activity are effective1 if maintained, but many patients do not achieve their goals with these measures alone and additional therapies are recommended if lifestyle changes have not resulted in significant benefit after 6 months and BMI remains ≥30 kg/m2.2
|
Obesity in Aotearoa New Zealand: Inequitably distributed and associated with social disadvantage Approximately one-third (30.9%) of adults aged 15 years or older, and one in ten children aged 2-14 years (9.4%), were reported as obese in the 2019/20 New Zealand Health Survey.3 New Zealand has one of the highest rates of obesity in the OECD. The highest rates occur among Māori (47.9%) and Pacific (63.4%) peoples, and those living in the most socioeconomically deprived areas, who are nearly twice as likely to be obese than those in the least deprived areas. |
Weight management in primary care
The role of a GP in weight management is two-fold.
Population-level change is necessary to reduce the obesogenic environment, and GPs can advocate to reduce the health determinants of obesity.
At the individual level, GPs have a unique opportunity to support patients in healthy lifestyles that prevent excessive weight gain and offer evidence-based interventions to those who would benefit from reducing weight.
A four-part approach to weight management is suggested in the New Zealand Weight Management in Adults guidelines:2
- Monitor. monitor weight and, if increasing, offer brief nutrition, physical activity and sleep advice.
- Assess. For patients who are obese or overweight with waist circumference >102 cm (male) or >88 cm (female), take a full history to identify clinical, social and behavioural factors that may affect their weight.
- Manage. Develop a weight management plan with individualised lifestyle interventions. Consider prescribing weight loss medication if lifestyle changes have not resulted in significant benefit after 6 months, and BMI remains ≥30 kg/m2;
- Maintain. Continue to support patients and monitor their weight, BMI, waist circumference, mental health and wellbeing.
Mrs B has a long history of unsuccessful attempts at lifestyle changes to lose weight and her current BMI and waist circumference make her a candidate for medication.
She has heard about Saxenda® from a friend and wants to know if it's right for her.
Liraglutide: What is it?
Liraglutide (Saxenda®) is one of four medications approved in New Zealand for the treatment of obesity.4 The others include orlistat (Xenical®), phentermine (Duromine®) and naltrexone hydrochloride/bupropion hydrochloride (Contrave® 8/90).
Liraglutide is a glucagon-like peptide-1 (GLP-1) analogue that is self-administered once daily by subcutaneous injection. It is supplied as a pre-filled pen containing 3 mL solution that can deliver doses of 0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg and 3.0 mg.
How liraglutide works
GLP-1 is an incretin hormone that acts in the brain to regulate appetite and satiety, and directly in the gut to delay gastric emptying and reduce food intake via vagal activity.5 Native human GLP-1 usually has a short half-life of <2 minutes due to rapid degradation in the blood by dipeptidyl-peptidase 4 (DPP4). Liraglutide has 97% structural similarity to native GLP-1, with two modifications that extend the half-life to approximately 13 hours after subcutaneous administration, allowing once-daily dosing.
What outcomes can patients expect?
Bodyweight reduction of 5-10% is expected with liraglutide at the target dose of 3.0 mg/day.
In clinical studies, including the SCALE programme (see below) and the S-LITE trial, liraglutide treatment resulted in 4-6 kg weight loss when used alongside diet and exercise interventions.6
It also improved comorbidities, including blood pressure, lipid and glycaemic profile, and sleep apnoea. Liraglutide induces predominantly visceral fat loss, rather than subcutaneous fat or lean mass loss, which contributes to its ability to improve cardio-metabolic comorbidities such as blood pressure, lipid and glycaemic profiles, and sleep apnoea.7
Clinical Evidence for Liraglutide: The SCALE Studies
The global SCALE (Satiety and Clinical Adiposity – Liraglutide Evidence in nondiabetic and diabetic individuals) programme involved multiple randomised, controlled trials investigating the efficacy of liraglutide in >5,000 participants who were obese (BMI ≥30 kg/m2) or overweight (≥27 kg/m2 to ≥30 kg/m2) with comorbidities.
All participants were advised to follow and maintain a 500kcal/day calorie deficit and to increase physical activity a minimum of 150 mins/week. Liraglutide was initiated at 0.6mg daily and up-titrated to the target dose of 3.0 mg/day.
In the SCALE-Obesity and Prediabetes trial, liraglutide recipients lost a mean 8.0% of bodyweight compared with 2.6% loss with placebo (p<0.001) after one year of treatment, and blood pressure, weight circumference and lipid levels were improved.8 Weight loss was sustained over 3 years, though patients typically lost the most weight in the first year.9 Some weight was regained after stopping treatment.
Other SCALE trials showed that liraglutide significantly reduced both bodyweight plus comorbidities in obese or overweight patients with poorly controlled type 2 diabetes (SCALE-Diabetes10), sleep apnoea (SCALE- Sleep Apnoea11), or hypertension or hyperlipidaemia (SCALE-Maintenance12).
Safety and side effects
Saxenda® should not be used in patients with hypersensitivity to liraglutide or any excipients.4
It is not recommended in patients with severe renal impairment (eGFR <30 mL/min/1.73m2), hepatic insufficiency, obesity secondary to endocrinological or eating disorders, or a history of major depressive disorder or other major psychiatric disorders.
It is not recommended in patients aged under 18 years, pregnant or breastfeeding patients, or those with severe gastrointestinal disease, medullary thyroid carcinoma or history of MEN2 syndrome.3
Like other GLP-1 RAs, liraglutide has been associated with acute pancreatitis and patients should be educated about signs and symptoms of this condition, and liraglutide stopped and not restarted if it occurs.
Liraglutide is indicated in other countries for the treatment of type 2 diabetes, but it is not registered for this indication in New Zealand. However, liraglutide can be used to treat overweight or obesity in New Zealand patients with type 2 diabetes.4
The most common adverse effects are gastrointestinal reactions including nausea, vomiting, diarrhoea, constipation, and headache which occurred in >10% of study patients. These effects were usually mild to moderate in severity, and were typically transient, resolving after a few weeks. Discontinuation due to these effects was rare. Injection site reactions may also occur; educate patients about using different injection sites each week.4
Who Is liraglutide for?
Liraglutide 3 mg is indicated, alongside a reduced-calorie diet and increased physical activity, for weight management in adult patients with a BMI of:
- ≥30 kg/m2 (obesity), or
- ≥27 kg/m2 to≥30 kg/m2 (overweight) with at least one weight-related comorbidity, such as dysglycaemia (pre-diabetes or type 2 diabetes mellitus), hypertension, dyslipidaemia, or obstructive sleep apnoea.4
A 3-month trial is suggested to identify responders. Treatment should be discontinued if the patient does not lose at least 5% of their initial body weight after 12 weeks of treatment with liraglutide 3.0 mg/day. For those who respond well, aim to continue treatment for at least 12 months.
As at November 2021: Liraglutide is not funded in New Zealand and the price varies based on individual pharmacy mark-up. Pen needles are supplied free of charge.
Liraglutide is safe for Mrs B to use, based on her medical history.
You explain that it is a daily injectable medication that is anticipated to result in an average bodyweight loss of 5-10% over time when used along with diet and lifestyle interventions.
You explain the cost. Mrs B indicates she is ok with this and wants to know more.
Starting Saxenda®: A how-to guide
Before starting treatment, discuss:
- Expectations. Measure baseline weight and calculate 5% weight loss. Remind patients that liraglutide is not a ‘quick fix’ and if 5% weight loss is not achieved after 3 months it may be stopped. Weight may be regained upon stopping.
- Administration. Provide training on how to use the pen for subcutaneous injection into the abdomen, front of the thighs, or upper arm. Offer resources and trouble-shooting support, including advice on what if a dose is missed (skip it if there are <12 hours until the next dose is due). Give a copy of the Consumer Medicine Information leaflet and direct patients to the How to use your Saxenda® pen YouTube video.
- Safe Storage. Unused pens must be kept in the fridge before they are first used. Pens in current use can be kept at room temperature (up to 30oC) for up to 30 days. Each 5-pack prescription comes with a pack of 100 NovoFine needles; store these safely out of reach of children and use a sharps container for disposal.
- Gastrointestinal adverse events (GI AEs). Advise patients that these are usually mild or moderate and transient, rarely leading to discontinuation. Recommend increasing water consumption, taking small, frequent meals, avoiding eating 1-2 hours before bedtime and reducing intake of alcohol and spicy foods. Gradual dose titration may help minimise the likelihood of GI AEs.
- Support. Direct patients to the SaxendaCare® phone line (0800 689 921) and website for further information.
- Monitoring. Plan a review at 3 months, and at any time before then if needed.
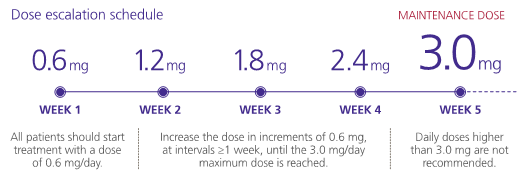
Liraglutide is supplied as a pre-filled pen containing a 3 mL solution that can deliver doses of 0.6 mg, 1.2 mg, 1.8 mg, 2.4 mg and 3.0 mg. All patients start with the lowest dose of 0.6mg, and progress weekly to reach 3.0 mg.
The rate of up-titration may be delayed by more than one week if adverse effects occur:
Together you look at how to use Saxenda®. You explain that it reduces appetite and increases satiety, and must be used alongside a reduced-calorie diet and exercise regimen. Together, you sketch out a diet plan that Mrs B feels able to manage.
You suggest that Mrs B begins with a trial aiming for 5% body weight loss after 12 weeks.
You warn that she may experience nausea initially but this should pass after 3-4 weeks, and advise her about strategies to minimise the GI side effects.
Mrs B is keen to start treatment and you arrange a prescription, plus a nurse appointment to go through the injection technique.
You book a 3-month follow-up visit but ask Mrs B to come back if she notices any side effects or has any concerns.
Practice points
- Liraglutide (Saxenda®) is a GLP-1 RA indicated for weight management, alongside lifestyle strategies, in patients who have obesity or overweight with comorbidities such as hypertension, prediabetes or diabetes, OSA or dyslipidaemia.
- Start with a trial of treatment for 3 months, aiming for 5% reduction in body weight; liraglutide can be stopped if this is not achieved. Ideally, continue liraglutide for at least 12 months.
- Mild gastrointestinal side effects are common but are usually transient and mild or moderate. Advise patients on strategies to minimise these.
This MedCase was written in 2021 by Dr Vicki Mount, MBChB FRNZGP DipPaeds MPH, with expert review by Dr Ryan Paul (BHB, MBChB, FRACP, PhD)
References:
Supported with an unrestricted educational grant from
Recognition of Learning Activities
Don't forget to log your time with The Royal New Zealand College of General Practitioners portal for recognition of learning activities.



