
Rosuvastatin is an HMG-CoA reductase inhibitor (statin) that reduces low-density lipoprotein cholesterol (LDL-C) levels. From 1 December 2021, it became fully funded for the management of cardiovascular disease and familial hypercholesterolaemia for patients meeting Special Authority criteria.1
This Medcase describes the safe prescribing of rosuvastatin for the prevention of cardiovascular disease.
Mrs R is a 73-year-old Māori woman. You last saw her 4 weeks ago for a repeat of her Symbicort inhaler and noted that her cardiovascular disease (CVD) risk assessment was overdue.
She was well with blood pressure 134/76 mmHg. She lives in a quintile 4 region, is an ex-smoker who quit over 20 years ago, and has no personal history of cardiovascular disease or familial hypercholesterolaemia. Her father had a myocardial infarction at age 59 years and her mother had a stroke at age 67 years.
Mrs R’s last blood tests were over 18 months ago, so you asked her to repeat them then return to discuss CVD prevention. In the meantime, Mrs R agreed to trial some lifestyle changes, including aiming for five 30-minute walks per week, replacing butter with olive oil for cooking and including 1-2 plant-based meals per week to reduce intake of saturated animal fats.
Today, Mrs R is feeling well. She tells you she has found the lifestyle changes mostly manageable and is enjoying trying new plant-based recipes. On examination, she has a regular radial pulse of 78 bpm, blood pressure 128/78 mmHg, weight 83.9kg, height 168.5cm, and BMI 29.6. Other blood test results, including HbA1c, TSH and renal function are normal.
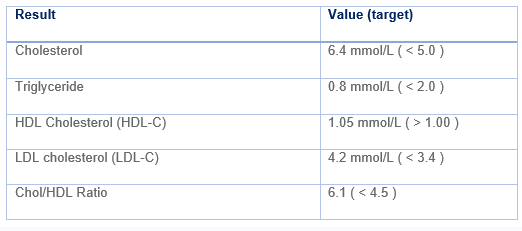
Her lipid profile:
You use the PREDICT tool to estimate Mrs R’s 5-year cardiovascular disease (CVD) risk, which is 14%.
What treatment strategy do you recommend?
Statins for primary prevention of cardiovascular disease
As per the New Zealand Cardiovascular Risk Assessment and Management guidelines, patients with a CVD risk of between 5 and 15% should be offered dietary and lifestyle interventions along with consideration of lipid-lowering or blood pressure-lowering pharmacotherapy, based on an individualised assessment of risks and benefits.2
A discussion of dietary and lifestyle modification is beyond the scope of this MedCase but further information can be found in the Cardiovascular Disease Risk Assessment and Management for Primary Care.2,3
Statins are the preferred first-line medications for lipid management, for both primary and secondary prevention of CVD events.2,4,5
Multiple, robust clinical trials have demonstrated that statins reduce the risk for nonfatal myocardial infarction, ischemic stroke, need for revascularization, and cardiovascular and all-cause mortality.5 They have also been shown to stabilize and even regress established atherosclerotic plaque. Each 1 mmol/L reduction in LDL-C confers a 25% reduction in relative risk of CVD events over 5 years.3 Reductions in LDL-C of >50% can be achieved in people with baseline LDL-C of >4 mmol/L, and CVD risk continues to decline commensurate with LDL-C with no evidence of waning risk reduction even at very low levels of LDL-C.2
In addition to diet and lifestyle changes, Mrs R could benefit from lipid-lowering therapy. Current guidelines recommend a target reduction in LDL-C of at least 40% i.e. target LDL-C of 2.52 mmol/L.
|
Getting to the heart of inequities in CVD prevention The burden of CVD and related mortality and morbidity is inequitably distributed in New Zealand. The rate of mortality from CVD is more than double for Māori than for non-Māori (RR 2.17, CI 2.08–2.26), and hospitalisations for CVD are over 1.5 times more common for Māori.6 Māori and Pacific peoples have greater exposure to CVD risk factors compared with other ethnic groups in New Zealand,7 yet are less likely to be prescribed statins for secondary prevention of CVD.8 The pro-equity Special Authority criteria for funding rosuvastatin aims to address these inequities by making rosuvastatin available as first-line therapy for any Māori or Pacific patient at risk of cardiovascular disease. |
You congratulate Mrs R on her success in implementing the dietary and exercise changes so far. However, you explain that further interventions are likely needed to reduce her cholesterol, and therefore her risk of heart attack or stroke.
Together, you use the CVDRA online decision aid (available via the practice management system) to illustrate that 14 of 100 people just like Mrs R will have a CVD event in the next five years. Reducing her LDL-C to 2.8 (a 40% reduction) would reduce this to 11 of 100 people.
Mrs R is open to the idea of medication, and asks which one you would recommend.
Which statin to start?
With the recent funding of rosuvastatin, there are now four fully-funded statins to choose from. Any statin may be started, and if treatment is tolerable and targets are achieved there is no need to change therapy.
All statins work to inhibit hepatic cholesterol synthesis, which involves a 30-step pathway.5 The rate-limiting step is the reduction of 3-hydroxy-3-methylglutaryl (HMG) to mevalonate. This step is regulated by the enzyme HMG acetyl coenzyme A (HMG-CoA) reductase, which is inhibited by statins. Additionally, statins up-regulate the expression of LDL-C receptors on hepatocytes, promoting clearance of LDL from circulation.5
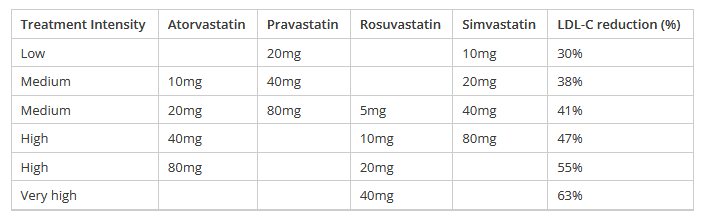
Approximate equivalence of statins (table adapted from He Ako Hiringa and bpacnz)
Rosuvastatin versus other statins
Rosuvastatin is the most potent statin for the reduction of LDL-C, requiring a lower dose to achieve therapeutic effect as shown in the table above. Higher doses of rosuvastatin allow LDL-C reductions not achievable with other statins, with comparable tolerability, though caution is advised; the 40mg dose is contraindicated in patients with a history of or risk factors for myopathy or rhabdomyolysis (see below).
Additionally, rosuvastatin produces greater increases in HDL-C and reduces triglycerides to an equal or greater extent than atorvastatin, simvastatin or pravastatin.11
Is my patient eligible for funded rosuvastatin?
Rosuvastatin is currently available under PHARMAC special authority criteria (Appendix 1) for prescription by any relevant practitioner, for patients meeting the eligibility criteria.1 A flow chart to determine funding eligibility can be found here. If the patient does not meet the funding criteria, consider prescribing a generic to reduce cost.
Points to note about the funding criteria are:
- Pro-equity criteria mean that all Māori and Pacific peoples with an increased CVD risk are eligible for funded first-line treatment with rosuvastatin;
- Patients on a maximum tolerated dose of atorvastatin and/or simvastatin must also have:
- a calculated risk of CVD of ≥15% over five years, and LDL-C level remains ≥1.8mmol/L;
- familial hypercholesterolaemia (Dutch Lipid Clinic Network score ≥6), and LDL-C level remains ≥1.8mmol/L;
- coronary artery disease, proven peripheral artery disease or ischaemic stroke, and LDL-C level remains ≥1.4mmol/L;
- a recurrent major cardiovascular event (myocardial infarction, ischaemic stroke, coronary revascularisation, hospitalisation for unstable angina) in the last two years, and LDL-C level remains ≥1.0mmol/L;
- The tolerability of a current statin is based on the prescriber’s clinical judgement;
- There is a waiver process for patients currently self-funding rosuvastatin who would have been eligible at the time of starting self-funding.
Clinical practice point: Lower target LDL-C for all secondary prevention patients
The PHARMAC special authority criteria recognise the value of achieving lower LDL-C for secondary prevention.
Target LDL-C is <1.4 mmol/L for patients with:
- established CVD, or
- recurrent major cardiovascular events, defined as myocardial infarction, ischaemic stroke, coronary revascularisation, hospitalisation for unstable angina).
Target LDL-C is <1.0 mmol/L for patients who have experienced a recurrent major cardiovascular event within the last 2 years.
Safety and interactions
Statins are generally well tolerated as a class. Adverse effects from rosuvastatin are usually mild and transient, with a discontinuation rate of less than 4% in clinical trials (similar to placebo).12 Serious adverse effects are rare and typically emerge in the first three months of treatment.7
The most common adverse effects include headache, dizziness, nausea, abdominal pain. Dose-related increases in liver transaminases and creatine kinase (CK) may occur.10
Myopathy and Rhabdomyolysis
Skeletal muscle effects of statins may range from myalgia to myopathy (muscle pain or weakness with a rise in CK) and rhabdomyolysis.10 Though rare, these effects are widely known and statin intolerance is often over-diagnosed. A stepwise approach and measurement of CK is recommended for patients reporting muscle pain (see your local HealthPathways or NZ Formulary for specific guidance).10
Diabetes
Rosuvastatin is associated with dose-related increases in HbA1c, contributing to a potentially higher risk of diabetes mellitus especially in patients at high risk of this condition.
However, the 2010 Cholesterol Treatment Trialists (CTT) meta-analysis found only one extra case or diabetes mellitus, defined as HbA1c of ≥50 mmol/mol on two occasions, per 1000 patients treated for one year with a statin.13
Thus, the absolute risk is low and must be considered against the benefits of reduced cardiovascular events which more than offset the diabetes effects.
Interactions
Statins have a range of potential interactions though, unlike simvastatin and atorvastatin, there are no clinically significant cytochrome P450 interactions with rosuvastatin. However, there are a number of important interactions based on rosuvastatin’s action as a substrate for transport proteins in the liver. See NZ Formulary and the Rosuvastatin Viatris Data Sheet for information on specific drug-drug interactions, including with commonly used medications such as clopidogrel, warfarin, colchicine and leflunomide.
Mrs R is eligible for Rosuvastatin as a first-line treatment based on her increased CVD risk and Māori ethnicity.
You explain that it is the preferred first-line medication because it has greater efficacy at lowering LDL-C and raising HDL-C than the other statins.
Together, you review the potential side effects and agree there is no current risk of interactions as Mrs R takes no other regular oral medications.
You briefly discuss the increase in diabetes mellitus risk, and agree to monitor HbA1c as part of routine testing. Mrs R is happy to start treatment.
Practical tips for starting rosuvastatin
Before starting treatment, ensure there are recent liver function tests and a CK level for a baseline reference; these should be monitored periodically and rosuvastatin dose reduced or withdrawn if there are persistent elevations in ALT or AST greater than three times the upper limit of normal.10
For hypercholesterolaemia, the usual dose range is 5-40mg once daily.10 No dosage adjustments are required for elderly patients or those with mild-to-moderate renal or hepatic impairment; specific recommendations can be found in the data sheet for patients with severe renal or hepatic impairments or genetic polymorphisms.
A lower starting dose of 5mg may be considered for some Asian patients (Filipino, Chinese, Japanese, Korean, Vietnamese or Asian-Indian origin), who may experience two-fold higher systemic concentrations of rosuvastatin. The 40mg dose is relatively contraindicated in these patients and up-titrations should be done with caution.
A response to treatment – a reduction in LDL-C – occurs within one week of starting treatment and 90% of the maximum response is evident after two weeks of treatment.
Rosuvastatin, like atorvastatin, has a long half-life and can be taken at any time of day, with or without food; pravastatin and simvastatin have shorter half-lives and are recommended for night-time dosing to ensure maximum effect on cholesterol synthesis, which peaks during overnight fasting.10
You prescribe Rosuvastatin 10mg for Mrs R and she agrees to start it tomorrow and to return in 3 months for review with a blood test beforehand.
She prefers to read printed information than websites, so you give her a printed copy of the Health Navigator information and ask her to phone the nurse if she experiences any new or concerning side effects.
Practice points
- Rosuvastatin is fully funded for eligible patients for primary and secondary prevention of cardiovascular disease according to Special Authority criteria.
- Statin safety profiles are similar, but efficacy differs; rosuvastatin elicits greater reductions in LDL-C along with equivalent or better changes in HDL-C and triglycerides, compared with other statins.
- Consider starting rosuvastatin at a lower 5mg dose in some Asian patients at risk of higher exposure to the drug.
- Monitor progress and titrate doses as required to achieve target LDL-C reductions.
- Note the lower LDL-C targets for patients requiring secondary prevention: <1.4 mmol/L for those with established CVD or recurrent cardiovascular events, and <1.0 mmol/L for those with a recurrent event within the last 2 years.
This MedCase was written in 2022 by Dr Vicki Mount ( MBChB FRNZGP), with expert review by Dr Chris Ellis (BM, MRCP (UK), FRACP, FACC, FCSANZ)
| Special Authority criteria for Rosuvastatin from 1 December 2021 |
|
Cardiovascular disease risk Either: 1. Both:
2. Both:
Familial hypercholesterolemia Both:
Established cardiovascular disease Both:
2. LDL cholesterol has not reduced to less than 1.4 mmol/litre with treatment with the maximum tolerated dose of atorvastatin and/or simvastatin.
Recurrent major cardiovascular events
PHARMAC Waiver for those currently self-funding who would have met the criteria above before starting Rosuvastatin. |
References:
Recognition of Learning Activities
Don't forget to log your time with The Royal New Zealand College of General Practitioners portal for recognition of learning activities.


