
This MedCase was first published in 2018. It was updated by Dr Hazel Fuiava, MBChB, Dip Paeds, FRNZCGP.
Adding sacubitril/valsartan to optimal chronic heart failure therapy provides better clinical outcomes for patients than can be achieved using treatment without this medicine.
This case study looks at the use of sacubitril/valsartan (brand name: Entresto), which is recommended as replacement for ACEi or ARB in patients with reduced ejection fraction heart failure who remain symptomatic on optimal therapy without Entresto.
Mr H, a 62-year-old Māori man, is at your clinic for a repeat of his usual medications for hypertension and heart failure.
You are reviewing your notes ahead of the appointment. Mr H was seen last month by Cardiology for follow-up of his longstanding heart failure, and the clinic letter mentions that he may be a candidate for Entresto.
Mr H also has mildly impaired renal function and type II diabetes for which he takes metformin 500mg BD. He is an ex-smoker and he drinks 1-2 cans of beer per night. His father died at age 58 from a heart attack, and his mother died in her 70s from COPD. His younger brother is also hypertensive.
Mr H is compliant with his medicines. He currently takes furosemide 40mg, perindopril 8mg, bisoprolol 10mg.
What is Entresto?
Entresto is an angiotensin receptor neprilysin inhibitor combination (an “ARNI”) medication for patients with heart failure with reduced ejection fraction (HFrEF)1.
- Valsartan is an angiotensin receptor blocker (ARB) that blocks the activity of angiotensin II at the receptor level. These drugs are well established for use in patients with heart failure.
- Sacubitril is a prodrug that ultimately causes vasodilation. It is converted to the active metabolite, LBQ657, which inhibits neprilysin, an enzyme that breaks down vasodilating natriuretic peptides such as brain natriuretic peptide [BNP].
Who should use it?
Sacubitril/valsartan is available under special authority for patients who meet the following criteria:
- Heart failure with NYHA functional class II, III or IV symptoms; AND
- Documented left ventricular ejection fraction of ≤35%, or an ECHO is not reasonably practical, and in the opinion of the treating practitioner the patient would benefit from treatment; AND
- Receiving concomitant optimal standard chronic heart failure treatments.
Mr H tells you he becomes tired and short of breath doing the supermarket shopping, and on his daily walks.
- He can walk approximately 100 metres on the flat before needing to stop. He is not breathless doing his usual daily tasks such as showering or doing laundry.
- He does not have chest pain.
- You assess him as having NYHA class III symptoms.
So, it seems he may benefit from this medication…but are there any contraindications?
Cautions and contra-indications
Contra-indications include:
- Patients with systolic blood pressure (SBP) ≤100mmHg as hypotension can be a side effect - Entresto should only be started when SBP >100.
- Patients with potassium >5.4mmol/L - wait until corrected or resolved to start Entresto, as hyperkalaemia can be a side effect.
- Patients with a history of angioedema related to previous angiotensin converting enzyme inhibitor (ACEi) or ARB therapy.
- Patients with severe hepatic impairment, biliary cirrhosis, or cholestasis.
- Concomitant use of an ACE inhibitor (increased risk of angioedema with sacubitril) or ARB:
- If stopping an ACEi and starting Entresto, wait at least 36hrs after the last ACEi dose before the first dose of Entresto.
- If stopping an ARB and starting Entresto, the first dose of Entresto can be taken when the next ARB dose would be due.
Caution is advised when prescribing in:
- Older patients, children, and women of childbearing age. Sacubitril/valsartan is Category D in pregnancy.
- Patients with NYHA class IV symptoms.
- Renal impairment - reduce starting dose to 24 mg/26 mg twice daily if eGFR less than 30 mL/min/1.73 m2; avoid in end-stage renal disease.
- Hepatic impairment - reduce starting dose to 24 mg/26 mg twice daily in moderate impairment; avoid in severe impairment.
Mr H’s blood pressure today is 134/76, and a quick search of MedTech shows his systolic blood pressure (SBP) usually ranges 120-140mmHg.
- His blood tests from two weeks earlier show eGFR 55ml/min and potassium 4.8mmol/L.
- His liver function tests are normal.
- He has tolerated perindopril for the past six years with no angioedema.
- You assess him as appropriate for sacubitril/valsartan.
But, he has been stable on his current regimen for some time… is it worth changing?
How effective is sacubitril/valsartan?
The additive effects of combination therapy with sacubitril/valsartan provide better clinical outcomes for patients with heart failure than can be achieved with current treatment.
The PARADIGM-HF trial2 evaluated patients with reduced ejection fraction heart failure and NYHA class II, III or IV symptoms who received either sacubitril/valsartan 200mg twice daily or enalapril 10mg twice daily.
- Sacubitril/valsartan significantly reduced death from cardiovascular causes and hospitalisation for heart failure compared with enalapril (see figure below).
- The NNT to achieve a reduction in cardiovascular deaths or hospitalisation for heart failure was 21 over 27 months.
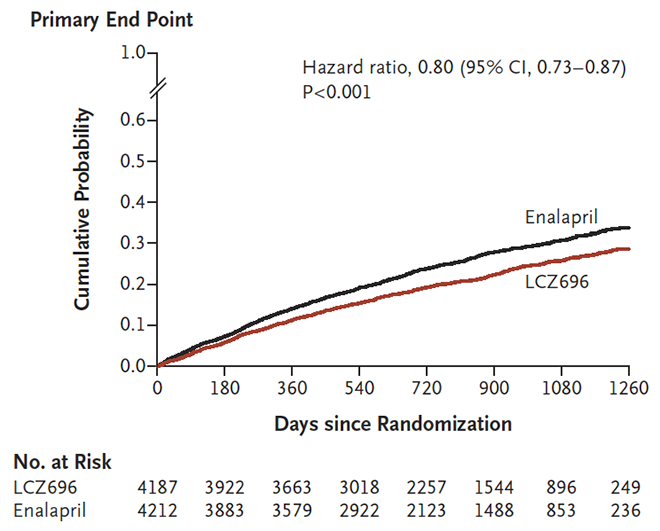
Figure 1: Primary endpoint (composite of death from cardiovascular causes or first hospitalisation for heart failure) in the PARADIGM-HF study2
Sacubitril/valsartan is recommended by European (ESC)3 and American4 heart associations as a replacement for an ACEi or ARB to reduce heart failure death or hospitalisation in patients who remain symptomatic despite treatment with an ACEi or ARB, beta-blocker and mineralocorticoid receptor antagonist.
In New Zealand, the Cardiovascular Disease Risk Assessment and Management for Primary Care consensus statement identifies patients with congestive heart failure as a high-risk group who require intensive management. New therapies for this patient population must be considered.
You consider the evidence and decide to start Mr H on sacubitril/valsartan.
How will you start this medication, and what monitoring is required?
How to start sacubitril/valsartan
It is recommended that you consider starting this drug in close consultation with your local heart failure service. (At any stage: consult your local Heart Failure Service for advice).
|
What will you warn Mr H about when starting the new medication?
Potential side effects
- symptomatic hypotension, especially in older patients;
- In patients aged >75 years, hypotension occurred more often with sacubitril/valsartan than with enalapril in the PARADIGM-HF trial (18% vs 12%)
- angioedema (0.4% vs 0.2%)
- diarrhoea
- headache
- gastritis
- worsening renal function.
Important interactions
There are several interactions to be aware of, and full information can be found on NZ Formulary. Some key drug classes to consider are:
- Statins: sacubitril/valsartan may increase the effect of statins.
- Drugs that may increase potassium and thereby increase the risk of hyperkalaemia: potassium-sparing diuretics, mineralocorticoids, potassium supplements.
- NSAIDs: risk of worsening renal function with coadministration, especially in elderly and volume-depleted patients (think: Triple Whammy).
- Sildenafil: risk of hypotension even with a single dose of sildenafil.
Practice points
- Sacubitril/valsartan is recommended as replacement for ACEi or ARB in patients with reduced ejection fracture heart failure who remain symptomatic on best therapy without it.
- When used appropriately along with optimal chronic heart failure therapy, it provides better prevention of cardiovascular death or hospitalisation for heart failure than ACEi or ARB alone.
- The most suitable patients are those with reduced ejection fracture heart failure, systolic blood pressure >100mm Hg, and eGFR >/= 30ml/min.
- Stop ACEi 36 hours before starting.
- Start at low dose and titrate to target 97/103mg BD.
- Monitor symptoms, blood pressure, renal function and potassium regularly.
- Liaise with your local heart failure service for advice.
References
Supported by
Recognition of Learning Activities
Don't forget to log your time with The Royal New Zealand College of General Practitioners portal for recognition of learning activities.



